Microscope creates first-ever 3D video of living cells inside the body using MATLAB
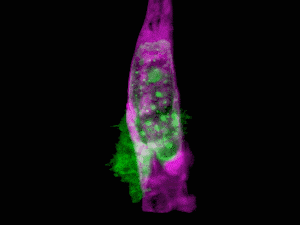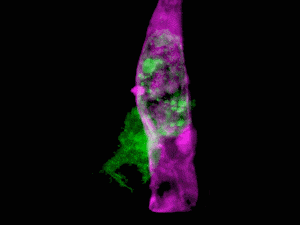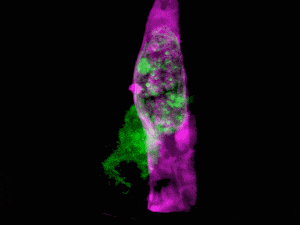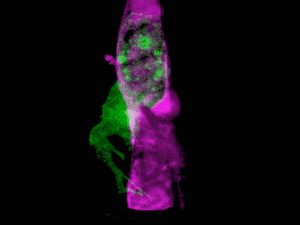
What do you get when you combine state-of-the-art microscopy with a technique used by astronomers to get a clear view of celestial objects through atmospheric turbulence? You get a new form of microscopy that lets you see stunning 3D movies of cells deep within living systems. For example, the video clip below shows a cancer cell (in green) invading a blood vessel (purple).
The AO-LLS (adaptive optics lattice light sheet) microscope that captured the video above was developed by a group of researchers led by physicist Eric Betzig. It merges lattice light sheet microscopy, which sweeps a thin sheet of light through a cell, with adaptive optics, a method used by telescopes to correct for visual disturbances as light travels through the atmosphere.
According to Engadget, “For the first time, scientists have peered into living cells and created videos showing how they function with unprecedented 3D detail. Using a special microscope and new lighting techniques, a team from Harvard and the Howard Hughes Medical Institute captured zebrafish immune cell interactions with unheard-of 3D detail and resolution. The tech has already yielded new insights on cell function and could transform our understanding of how organisms function at the smallest scales.”

Organelle morphologies and dynamics in zebrafish. (A) Computationally separated neural progenitor cells in the brain of a developing zebrafish. (B) Changing morphologies of the organelles in the specific cell outlined in (A). (C) MIP views within the eye of a zebrafish embryo. (D) Six images from Movie (shown below). (E) Correlation between nuclear volume and total cell volume in the eye and ear. (F) Different morphologies of trans-Golgi near the spine of a zebrafish embryo. Image Credit: Betzig et al./Science 2018
Two main challenges in imaging living cells
The AO-LLS microscope overcomes the two biggest challenges in imaging the cells in living tissue. The first challenge comes from the illumination method: The light is typically so bright and harsh that it can damage the cells the scientists are trying to study.
“Life wasn’t really evolved to take that kind of abuse. If you don’t outright kill the cell, you’re always left wondering, ‘What did I do to this poor thing, and am I really seeing it the way it normally is?’” says Betzig.
LLS significantly decreases the amount of light used needed to image cells. LLS microscopy isn’t new, but the resolution decreased as the light moved deeper into multicellular systems.
Betzig told Nature, “It was incapable of imaging deep within a sample, because the deeper light penetrates the tissue, the more it ‘warps’ or bends. As a result, though it could image cells on cover slips, it could not do so, say, deep within a growing embryo. Now, that limitation has been lifted.”
That’s where the second challenge comes in. The researchers needed to account for the distortion that occurs as you try to image cells deep within the sample.
According to National Geographic, “To help correct for this, Betzig borrowed a technique from astronomers called adaptive optics. With telescopes on Earth, our planet’s atmosphere similarly distorts images taken of distant objects in space. Adaptive optics measures that distortion and corrects for it, offering clear, unwavering pictures of stars, galaxies, and other cosmic objects.”
The results are stunning, enabling scientists to see living cells in their natural state. The work was published in Science and includes a number of videos that show how cells function in their natural environment. For this study, the cells were those of a zebrafish embryo.
The microscope
The AO-LLS microscope is far from a simple set-up. Betzig estimates the microscope costs are near $800,000 in components alone. The parts list includes 35 lenses, 29 mirrors, two lasers, four cameras, and a vibration-dampened optical table.
In addition to being hardware intensive, AO-LLS is computationally intensive. At each time step, the changes in cellular structure are identified, tracked, and measured. Calculations also correct for the physical movement of the zebrafish embryos.
MATLAB and Image Processing Toolbox were used to segment, track, and measure cells and cellular structures in three dimensions. The research team used Image Processing Toolbox filters and morphology functions to clean up the segmentation of individual cells and to identify nuclei of each cell and other features. The researchers created their own custom MATLAB functions to track the cell structures.
One of the many videos captured by the researchers is linked blow:
Video Credit:
T. Liu et al./Science 2018
More on microscopy
If you’d like to see a different type of microscopy that utilized deep learning, check out this AI-augmented microscope developed by a multidisciplinary team of UCLA researchers.
Here’s an article on underwater microscopy that was used to study coral bleaching at Australia’s Great Barrier Reef.
If you are working on microscopy, leave a comment about your project below.
 Cleve’s Corner: Cleve Moler on Mathematics and Computing
Cleve’s Corner: Cleve Moler on Mathematics and Computing The MATLAB Blog
The MATLAB Blog Guy on Simulink
Guy on Simulink MATLAB Community
MATLAB Community Artificial Intelligence
Artificial Intelligence Developer Zone
Developer Zone Stuart’s MATLAB Videos
Stuart’s MATLAB Videos Behind the Headlines
Behind the Headlines File Exchange Pick of the Week
File Exchange Pick of the Week Hans on IoT
Hans on IoT Student Lounge
Student Lounge MATLAB ユーザーコミュニティー
MATLAB ユーザーコミュニティー Startups, Accelerators, & Entrepreneurs
Startups, Accelerators, & Entrepreneurs Autonomous Systems
Autonomous Systems Quantitative Finance
Quantitative Finance MATLAB Graphics and App Building
MATLAB Graphics and App Building








댓글
댓글을 남기려면 링크 를 클릭하여 MathWorks 계정에 로그인하거나 계정을 새로 만드십시오.